This information should be read in conjunction with the Summary of Product Characteristics (SmPC) issued by each vaccine manufacturer
What is pneumococcal disease?
Pneumococcal disease is a bacterial infection caused by streptococcus pneumoniae of which there are more than 90 serotypes. The organism is frequently found in the upper respiratory tract of healthy individuals worldwide. It has been estimated that carriage of the bacteria may range from 10% of adults to 50% of children attending day care facilities.
Over the years streptococcus pneumoniae has become resistant to many medications making the treatment of pneumococcal infections much more difficult. Prevention of disease through vaccination is now more important than ever.
What are the symptoms of pneumococcal disease?
Pneumococcal infection is responsible for 50% of community acquired pneumonia and bacteraemia where the overall mortality rate can be as high as 25%. It can also cause a wide variety of other infections including sinusitis, osteomyelitis, bronchitis and otitis media.
Who is most at risk of pneumococcal disease?
Pneumococcal disease can lead to significant morbidity and mortality, particularly amongst the very young, the very old, those with impaired immunity and those with anatomic or functional asplenia.
How is pneumococcal disease transmitted?
Transmission requires close contact with cases or carriers and is by droplet infection. Person-to person transmission of the organism is common. The incubation period can be difficult to determine but can be as short as 1-3 days.
Which pneumococcal vaccines are recommended in Ireland?
Pneumococcal conjugate vaccines and pneumococcal polysaccharide vaccines are licensed in Ireland
Pneumococcal conjugate vaccine (PCV)
PCV 13 - Prevenar 13 - contains polysaccharide from 13 of the most common capsular types (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F). It is recommended for the routine vaccination of all children born on or after 1st October 2010. This replaced PCV 7 (Prevenar 7) the pneumococcal conjugate vaccine introduced into the routine childhood immunisation programme in September 2008.
Pneumococcal polysaccharide vaccine (PPV)
This vaccine contains purified polysaccharide from 23 of the most common capsular types of streptococcus pneumoniae. This vaccine is recommended for those aged 65 years and older and "at-risk" adults and children over 2 years of age. To find out if vaccination is required please consult your copy of the PPV23 algorithm (PDF).
What impact have PCV vaccines had?
PCV7 vaccine – containing an antigen from 7 of the most common capsular types (4, 6B, 9V, 14, 18C, 19F, 23F) was introduced into the routine primary immunisation schedule in September 2008, with a catch up programme for children under 2 years of age.
Since then the burden of notified confirmed cases of IPD has been reduced by 4%. The greatest reduction has been seen in young children, particularly in those aged less than 5 years (See Figure 1).
Figure 1
Age-specific incidence rates of confirmed invasive pneumococcal disease (IPD) notifications, 2008-2016
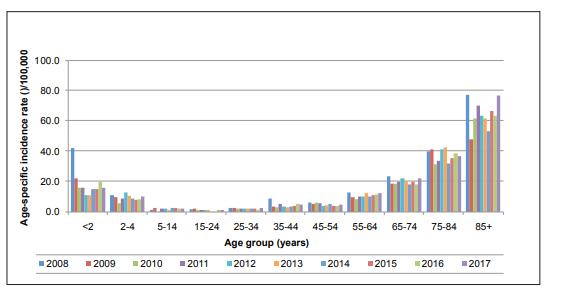
Source: Health Protection Surveillance Centre (HPSC)
The decrease in this age group can largely be attributed to a 98% decline in Invasive pneumococcal disease (IPD) due to serotypes covered by PCV7 between 2008 and 2016.
A decline of 50% in notifications of disease caused by the additional serotypes in PCV13 was also observed.
In December 2010 PCV13 vaccine replaced PCV7 in the Irish childhood immunisation programme. PCV13 includes antigens from the seven serotypes contained in PCV7 plus six additional serotypes. Pneumococcal conjugate vaccines reduce the rates of nasopharyngeal colonisation by vaccine serotypes, thus decreasing the potential for transmission from vaccinated to unvaccinated persons.
Who should not receive PCV13?
Children should not get PCV13 if they had a serious (life -threatening) allergic reaction to a previous dose or any of its constituents.
When should vaccination be postponed?
As for any vaccine PCV vaccination should be postponed in those individuals who have an acute febrile illness.
What should people expect after vaccination with PCV13?
In studies, most reactions after PCV13 were mild. They were similar to reactions reported after PCV7, which had been in use since 2000 in the US. Reported reactions varied by dose and age, but on average:
- About half of children were drowsy after the shot, had a temporary loss of appetite, or had redness or tenderness where the shot was given.
- About 1 out of 3 had swelling where the shot was given.
- About 1 out of 3 had a mild fever
Who should be vaccinated with PPV23?
Pneumococcal disease is a very serious disease. It is a major cause of illness and death, particularly amongst the very young. Those with the following conditions should be vaccinated with PPV23.
Everybody aged 65 years and over and everybody aged 2 years and over with;
- Diabetes
- Chronic lung, heart, liver, or kidney disease
- Chronic neurological disease
- Children aged over 2 years and under 5 years of age with a history of invasive pneumococcal disease
- Coeliac disease
- Down Syndrome
- Cochlear implants or are about to get cochlear implants
- Immune deficiency because of a disease or treatment, including cancer patients
- HIV infection
- Absent spleen or a non-functioning spleen
- CSF leaks, either congenital or complicating skull fractures or neurosurgery
- Intracranial shunt.
PPV23 vaccination is not recommended for healthy children and adults as they are at low risk of pneumococcal disease
Who is eligible for a free PPV23 vaccination through the HSE vaccination programme?
The following people are eligible for a free Pneumococcal PPV23 vaccination*:
- 65 years or older
- aged 2 or over and in an at-risk group
People with one or more of these health conditions can get a free Pneumococcal PPV23 vaccine.
Everybody age 2 years and over with:
- diabetes
- chronic lung, heart, liver or kidney disease
- chronic neurological conditions
- coeliac disease
- Down syndrome
- cochlear implants (or about to get cochlear implants)
- a weak immune system because of a disease or treatment, including cancer patients
- HIV
- problems with their spleen or if they've had their spleen removed
- cerebrospinal fluid leaks
- a brain shunt
Children between 2 and 5 who have a history of pneumococcal disease should get the pneumococcal vaccine.
*Eligible for the free vaccine however, the administration of the Pneumococcal vaccine in a Primary Care setting is only free for those with Medical Cards or GP Visit Cards. Those without one of these cards are required to pay a consultation fee.
Who should not receive PPV23?
PPV23 should NOT be given to those with a history of anaphylaxis to any of the vaccine constituents.
Precautions: Acute severe febrile illness - defer until recovery.
Pregnancy: PPV23 can be given if there is an urgent need for protection.
Are there any side effects from vaccination?
The most commonly reported adverse reactions are localised redness and swelling at the injection site (>10%). If the vaccine is administered intradermally then a severe local reaction may occur.
How often is vaccination with PPV required?">How often is vaccination with PPV23 required?
| Revaccination with PPV23 can produce severe local reactions especially if given within 5 years of previous injection. |
Aged 65 years and older
One PPV23 pneumococcal vaccine is recommended for anyone aged 65 years or older irrespective of immune status.
A once only booster vaccination is recommended 5 years after the first vaccination for those who received a previous dose at less than 65 years of age.
Aged less than 65 years of age
One booster vaccination is recommended 5 years after the first vaccination for those whose antibody levels are likely to decline rapidly e.g. for those who have asplenia, hyposplenism, immunosuppression including HIV infection, chronic renal disease, nephrotic syndrome or renal transplant.
Patients with these conditions who received PPV23 at less than 65 years of age require one further PPV23 booster at or after 65 years of age (five years after the previous dose)
If PPV23 was given during chemotherapy or radiotherapy a further dose vaccine is recommended 3 months after treatment.
Schedule for administering both PCV13 with PPV23 vaccines
Some patients at high risk of invasive pneumococcal disease are recommended both the PCV13 vaccine and the PPV23 vaccines.
PCV13 vaccine should always be given first followed by PPV23 vaccine after a time interval of 2 months to provide maximum immunological efficacy.
If the PPV23 vaccine is given first the PCV13 vaccine should not be given for an interval of one year.
When is a 3rd dose of PPV23 required?
Adults whose antibodies are likely to decline rapidly should receive two doses of PPV23 while aged less than 65.
They will need a third dose of PPV23 when they turn 65 and when at least five years have passed since their last dose of PPV23.
Depending on age and risk factors a person may require 1, 2 or 3 doses of PPV23.
If an immunosuppressed person aged 65 or over has received PPV23 on or after their 65th birthday do they need a further dose of PPV23 after 5 years?
No. A person aged 65 or over who is immunosuppressed should only receive one dose of PPV23. This is because the immune response to the vaccine declines as people older.
Can PPV23 vaccine be given at the same time as the influenza vaccine?
Yes. Pneumococcal vaccine may be given at the same time as influenza vaccine but at a different site.
As there is considerable overlap in the target groups for both vaccines, it is appropriate to offer PPV23 to patients (if indicated) when they attend for their influenza vaccine.
You can download a copy of the PPV23 algorithm for people aged 65 years and older (540kb) and the PPV algorithm for people aged 64 years and younger. (547kb) This outlines the groupings that need PPV23 and how many doses they require.
Information materials
- Leaflet - English (863kb), Irish (872kb), Arabic (712kb), Simplified Chinese (602kb), French (126kb), German (98.8kb), Lithuanian (99.5kb), Polish (100kb), Portuguese (96.1kb), Russian (105kb), Romanian (99kb)
- Poster - English (904kb), Irish (902kb)
- Frequently Asked Questions for those giving PPV23 vaccine (862kb)
Find out more
Back to top
This page was updated on 4 October 2024.